NTI get HREC approval to extend Phase II/III ASD Trial to adults
Today, our biotech Investment, Neurotech International (ASX: NTI) announced Human Research Ethics Committee (HREC) approval to extend its current Phase II/III clinical trial in Autism Spectrum Disorder (ASD) patients to allow for patients who turn 18 years of age to remain on treatment with NTI164 during the extension phase of the trial for up to 54 weeks of total treatment.
Previously, NTI’s Phase I/II ASD (Autism) trial showed no serious adverse events across 52 weeks.
With a strong safety profile - today’s extension approval is similar to what happened in NTI’s Phase I/II trial which was also extended - we think that those carers with children on the trial have seen a marked improvement in their children’s symptoms and are comfortable with the safety of NTI’s treatment.
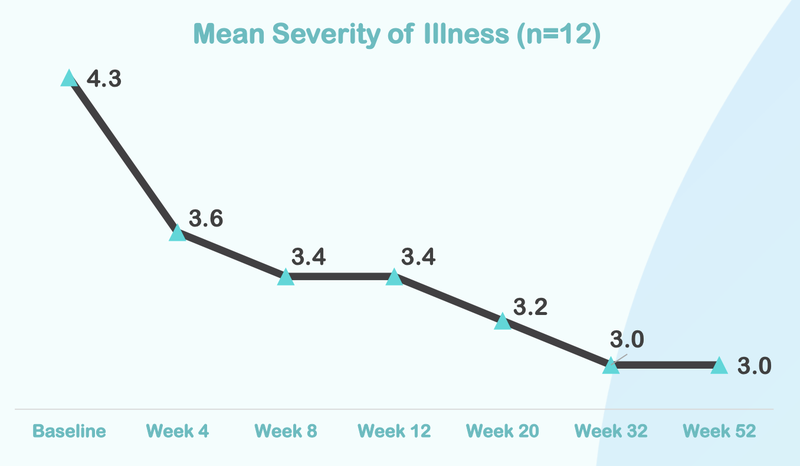
Below is some of the data from NTI’s Phase I/II trial.
From this chart you can see that the severity of illness for children with Autism moved from “Markedly” to “Mild”:
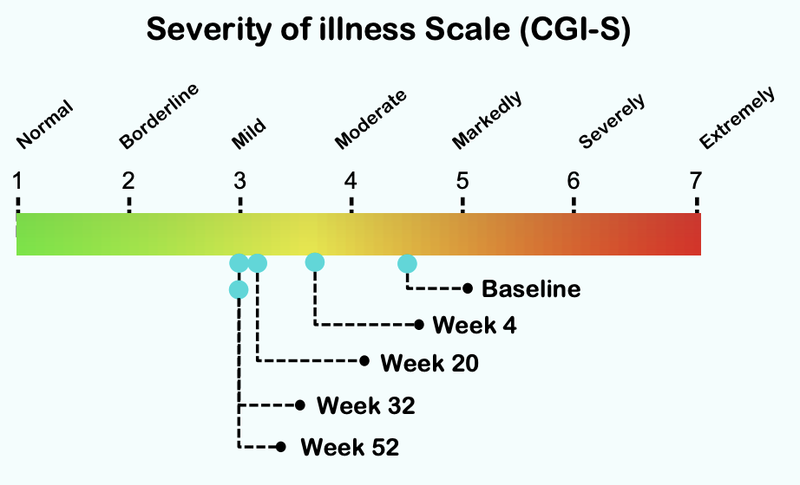
Here is how the treatment reduces over time, with significant improvement after just four weeks of taking the treatment:
Also of note, is that the trial remains on track to report results in Q1 CY2024, which could further cement NTI’s already promising data showing that its treatment for ASD is both safe and effective.
This is earlier than we expected.
There’s a large addressable market on offer for NTI if it can commercialise its treatment for ASD - and we think it could become an important part of the overall care for ASD sufferers, which is 1 in 100 children.
Catch up on NTI by reading our latest notes:
Introducing our new Portfolio Addition: Neurotech International (ASX: NTI)
NTI’s clinical trial meets primary endpoints for neurological disease
What’s next for NTI?
Catalyst #2: 🔄Phase I/II Rett Syndrome clinical trial (results in Q1 CY2024) - a trial on another child neuropsychiatric disorder called Rett Syndrome - Neuren Pharmaceuticals re-rated ~1300% on commercialisation of its treatment for this disorder. We think NTI could be safer than Neuren’s treatment and if it works better or similar, hopefully, re-rate accordingly.
Catalyst #3:🔄 Phase II/III trial for Autism Spectrum Disorder
We’re now expecting results in Q1 CY2024, after today’s announcement which was earlier than we originally thought.






