BPH investee Cortical Dynamics applies for BARM FDA approval
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
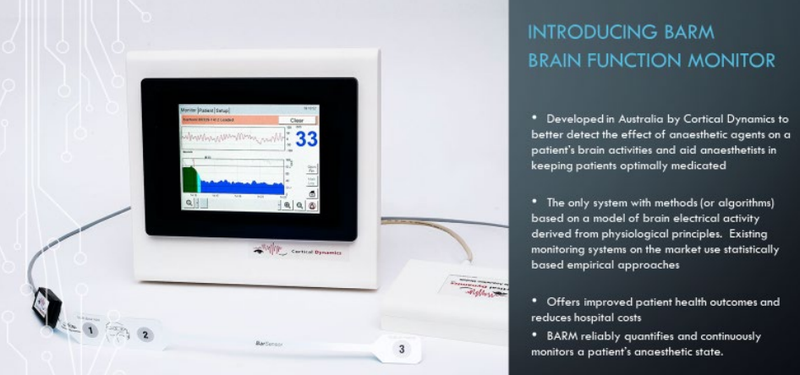
BPH Energy (ASX:BPH) investee Cortical Dynamics has begun the filing process for its BARM technology (brain anaesthesia response monitor) in the US with the filing of an FDA 510K.
The Food and Drug Administration (FDA) is the federal agency of the United States Department of Health and Human Services which regulates the sale of medical device products (including diagnostic tests) in the US, while also monitoring the safety of all regulated medical products.
FDA approval is a necessary precursor for sales of BARM to commence in the US.
Cortical already has achieved both CE (Europe) and TGA (Australian) registration and is currently awaiting final approval of the company’s registration application to the Korean Ministry of Food and Drug Safety.
Having achieved registration in Europe and Australia, one would expect the chances of receiving FDA approval are relatively high.
The US is a large market, and this would be a significant development for Cortical, and indeed BPH given its 16% interest in the group.
Shares in BPH double on back of strong news flow
Cortical Dynamics is finishing the fiscal year in strong fashion, having announced last week that it had entered into a non-exclusive Licence and Co-operation Agreement with Philips Healthcare North America Corp.
The agreement will enable Cortical to interface its BARM into the Philips IntelliVue and Patient Information Center (PIC iX) Monitoring Systems using the IntelliBridge integration product line.
Royal Philips (NYSE:PHG; AEX:PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.





