DXB announces approval for Phase III COVID-19 trials
Last month, we announced our very first Biotech Pick of The Year 2021 and investment in Dimerix Ltd (ASX:DXB).
DXB has developed a treatment for inflammatory diseases such as kidney and respiratory diseases and is busy advancing multiple near-term Phase 3 clinical studies.
The main reason that we invested in DXB is the fully funded Phase III clinical trials for FSGS (a rare kidney disease) - we like to call this our “main bet”.
DXB also has two COVID-19 treatment studies that are progressing in Phase III clinical trials in the near term.
These are what we call our "DXB side bets", and we hope that a successful outcome in either of these studies will be positively received by the market.
IMPORTANT: DXB is hosting its annual shareholder meeting TODAY at 2pm AEST. DXB Managing Director Nina Webster will be running through a presentation and answering shareholder questions.
If you want to attend DXB’s AGM shareholder presentation (virtually) you can do so via this meeting link.
On Friday last week, DXB announced progress in its COVID-19 treatment trials. The main drug regulator in India has recommended approval for DXB’s Phase III clinical trials on COVID-19 treatment, specifically for patients with respiratory complications intended for hospitalisation — its CLARITY 2.0 study.
This was the final regulatory approval required to commence recruitment for the study and the first patient is expected to be dosed in the next few weeks.
A total of 600 patients will be recruited into the trial. There will also be a safety analysis conducted on an initial group of 80 before seamlessly continuing to enrol the full cohort.
If this trial is successful, it will pave the way for future regulatory approvals in other jurisdictions like the US and Europe, without the need for another clinical trial.
What is the CLARITY 2.0 Study?
The Controlled evaLuation of Angiotensin Receptor Blockers for COVID-19 respIraTorY disease or CLARITY will evaluate whether DXB’s drug DMX-200 will be able to treat patients diagnosed with COVID-19 who are intended for hospital admission.
(They really tried to jump through some hoops with that acronym...)
In late 2020, DXB entered into an agreement with the NHMRC Clinical Trials Centre to undertake the study. Ethics approval was then received in March. And after delays to the approval process — primarily due to the COVID outbreak in India — the study is now back on track with the first patient expected to be dosed in the next few weeks.
Professor Meg Jardine of the Clinical Trials Centre will undertake a study to evaluate whether the DXB’s anti-inflammatory drug DMX-200 will be better at treating COVID-19 (with a level of statistical significance) than a placebo.
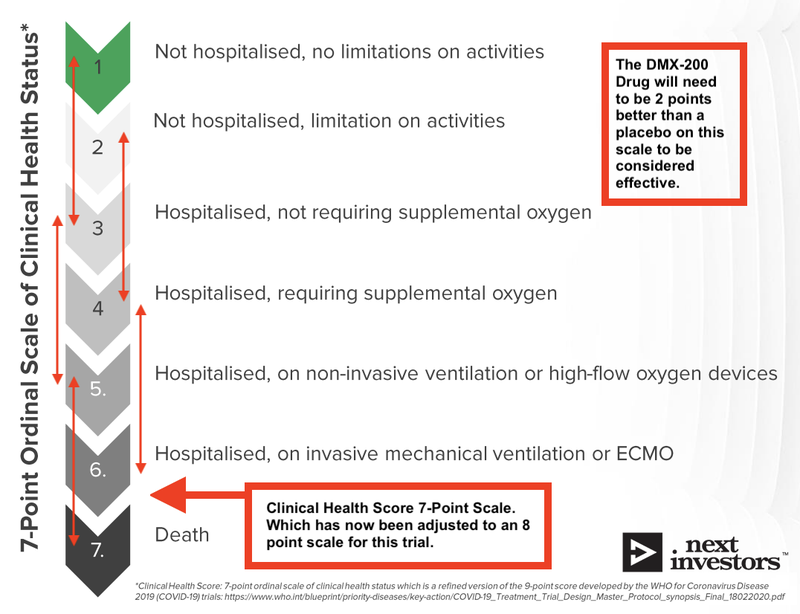
To evaluate the success, DXB’s drug will be measured on a 7 point scale (which is set out by the World Health Organisation, updated to 8 points in Friday’s announcement), and benchmarked against the effects of the placebo in 600 COVID-19 patients in India.
DXB is looking for a statistically significant result between DMX-200 and the placebo. The primary endpoint for DXB is that the DMX-200 drug needs to be 2 points higher than the placebo.
Here is an example of the seven point scale, which has been updated to 8 points for this particular trial:
Recruitment
Patient recruitment is a critical stage for a biotech company doing clinical trials. This is where the company undertaking the research will reach out to patients that have the relevant disease (in this case COVID-19) and enrol them in the study.
For early stage biotech investors, recruitment can be a risk point and delays may happen. However, there are mitigating factors that we think will reduce the recruitment risk in this particular trial.
The COVID-19 situation in India is reaching a stage of endemicity. Which means that the disease is not going away anytime soon but the effects will be less devastating. Like the flu, there will likely be repeatable outbreaks of COVID-19 in India, correlated with the seasons.
With winter steadily approaching in India, unfortunately we anticipate COVID-19 cases and hospitalisations to go up. However for DXB this means there will be more patients available to recruit and allow the company to move closer to helping those suffering.
Currently 16.3% of the Indian population is fully vaccinated with 46.1% with their first dose.
Our investment in DXB
Building on our own research, we are leveraging the knowledge of one of Australia’s most successful small cap biotech fund managers, the Merchant Group. We will be following the lead of the group’s early stage biotech fund, investing in high potential, early stage ASX listed biotech companies.
As the Next Investors Biotech Pick of The Year, DXB is also our first Finfeed portfolio investment.
If you would like to follow Finfeed, and our growing portfolio of biotech investment you can sign up here: https://finfeed.com/.
Here are the project milestones we expect to see for our “DXB Side Bet”
CLARITY 2.0: Respiratory Complications
✅ Study Announced
✅ Ethics Approval Granted
🔄 Regulatory approval by DCGI
🔲 Patient Recruitment Update 1
🔲 Safety Threshold Met (80 Patients)
🔲 Patient Recruitment Update 2
🔲 Patient Recruitment Update 3
🔲 CLARITY Phase III Study Complete
🔲 Primary Endpoint Reporting
🔲 Emergency use granted
🔲 Drug Commercialised
🔲 New Milestones Added
REMINDER: About Dimerix (ASX:DXB)
For this investment in DXB we are taking our lead from early stage biotech experts and one of the most successful small cap biotech fund managers in Australia, Merchant Group.
The group’s new Merchant Biotech Fund has now made its first investment: in DXB... so we did too.
We have been investing in ASX small caps for many years, and we get shown a LOT of investment opportunities from a LOT of different people. We say no to most of them.
Over the last 12 months, biotech expert and fund manager at Merchant Group, Andrew Chapman, told us a bunch of times that we should invest in another company called BARD1 Life Sciences (we said no each time).
It then went from 60¢ to almost $4 in a few weeks.
Before that Merchant also told us to look at Race Oncology (we didn’t invest in that one either) and it has been one of the ASX’s most successful biotechs in the last few years.
We get shown over 400 potential investments per year and we say no to the majority of them...
However, we have observed that the companies that Merchant recommends have gone on to perform extremely well.
So when Merchant approached us a few months ago to join in the due diligence process looking at DXB with them, this time we decided to get involved.
Merchant tells us that DXB has one of the cheapest market caps they have seen for a biotech company that is entering Phase 3 trials. Merchant invested $6M in cold hard cash into the recent DXB placement, making DXB the FIRST investment in their NEW early stage Merchant Biotech Fund.
SUMMARY: 9 Reasons we have invested in DXB
- We are following the lead of experts at the Merchant Group, DXB is the first investment in their new early stage biotech fund and they have an excellent track record.
- We have spent some time with DXB CEO Nina Webster and are very impressed with her industry experience in commercialising biotechs
- DXB MAIN BET: Addresses an inflammatory kidney disease to address a $1 billion market if successful. Phase III trial results are due in 12 months with investor speculation building in the lead up to the results.
- DXB SIDE BET #1: Phase III trials of lung inflammation treatment for patients in hospital from COVID — results due in a few months.
- DXB SIDE BET #2: Phase III trials treatment for patients with pneumonia associated with COVID — results due in a few months.
- Share price consolidated during 2021 and $20M in the bank — ready for next leg up with funding.
- We think life science and biotech will be a big theme over the next few years.
- Our new biotech expert advisor tells us the DXB science is very good.
- We think that if it proved the treatment, DXB could be acquired by big pharma for hundreds of millions.
You can read our deep dive into each of the above nine reasons in our original launch article for DXB here.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.






