Noxopharm receives go-ahead for NOX66 trials
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
Noxopharm (ASX:NOX) released a brief on Wednesday morning confirming its inaugural clinical study of experimental anti-cancer product, NOX66 is on schedule to open in December 2016.
This upcoming study had been flagged and as such is unlikely to be seen as market moving news, however of importance is the fact that receipt of approval has allowed NOX to release details of the study.
Management highlighted that the main purpose of the study was to test the safety and clinical benefits of using NOX66 in conjunction with carboplatin therapy in patients with late stage solid cancers.
What is NOX66
To provide some background, NOX is primarily focused on the development of drugs to address the problem of drug resistance in cancer cells and its NOX66 product is the first to enter the trial stage with later generation drug candidates under development in a research and development program.
In essence, NOX66 is an innovative dosage formulation of the experimental anti-cancer drug idronoxil, developed specifically to protect idronoxil from being inactivated in the human body by Phase 2 metabolism.
By ensuring that most idronoxil administered remains in an active form NOX66 is aiming to permit the drug to carry out its task of cancelling pro-survival mechanisms in cancer cells that allow the cells to resist the killing effects of chemotherapy is an radiotherapy
NOX will be looking to prove that its product will enable drug-resistant cancers to respond to carboplatin in a meaningful and well-tolerated way. The universe of patients trialling the drug will be those who have exhausted all standard treatment options so that any further response to carboplatin normally wouldn’t be anticipated.
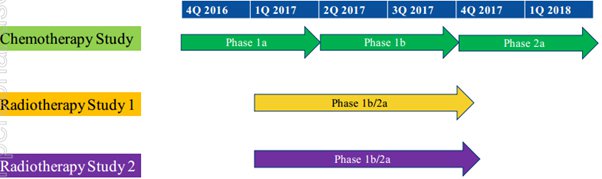
The next 12 months promises to be an exciting period for NOX as a number of its milestones are completed. These include the following:
Applications across a broad range of cancers
NOX can potentially cover a broad spectrum of cancers because it is used in conjunction with the carboplatin therapy, and patients will include those suffering from breast, lung, ovary, prostate, head and neck cancers.
One of the key factors that will be under scrutiny in the course of the study is whether a meaningful response can be gained by using a lower than normal dosage of carboplatin.
The challenge facing the medical fraternity at present is the fact that traditional chemotherapy treatment kills both cancer and healthy cells.
NOX Chief Executive, Doctor Graham Kelly summed up the end game in saying, “The aim is to boost the killing effect of chemotherapy on cancer cells without having any effect on healthy cells, and to do this with the dosage of a chemotherapy drug such as carboplatin that should not cause any side-effects”.
The trials will start with three monthly treatments with a low dose of carboplatin followed by three monthly treatments of a standard dose of carboplatin. The fact that patients will be monitored each three months for tumour response could suggest a reasonable lag time before NOX could be able to provide some meaningful data.
Potential opportunity to quickly transition to Phase 2 trial stage
Importantly though, the study has an adaptive design meaning that in the event of significant tumour responses after either the low or standard carboplatin dose, the study can be expanded immediately into a Phase 2a arm through the recruitment of a further 20 patients.
Such a development would be a game changer for NOX. The other factor to bear in mind is that the study is being conducted in Georgia because of the anticipated speed of patient recruitment, backed up by a high standard of healthcare and FDA audited clinical trial sites.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.



