Neurotech’s trial-backed device a new frontier for kids with autism
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
Perth-based medtech junior, Neurotech International (ASX:NTI), has developed a neurofeedback device that helps children with autistic spectrum disorders (ASD) to focus and positively engage with the world around them.
Autism, or ASD, is a developmental disorder that affects around 1 in 160 people worldwide — or, more than 40 million people across the globe. Autism can present in a variety of ways and impact areas such as balance, behaviour, social skills and the ability to communicate.
An independent report estimated the annual economic cost of ASD of between A$8.10 billion and A$11.26 billion in 2011, in Australia alone, highlighting a critical need for new treatments and solutions.
Neurotech’s tool, which is called Mente Autism, is the first and only regulated medical device for the management of ASD in a home setting.
NTI’s Mente Autism device was profiled by 9News this week:

Mente Autism helps relax the minds of children on the autism spectrum, and comes in the form of a headband worn for 40 minutes each morning.
Taking into account the unique brainwave signature of children with autism, Mente’s proprietary technology creates a personalised neurofeedback therapy that is specific to the user.
It connects to an app and analyses brainwaves in real time, creating tailor-made auditory stimulation that is played through earphones, which has the effect of regulating certain brainwaves that are commonly associated with autism.

The result is improved communication skills and social interaction, heightened focus and relaxation.
It should be noted, however, that this is an early stage tech company and success is no guarantee. Investors should seek professional financial advice before making an investment.
How it works:
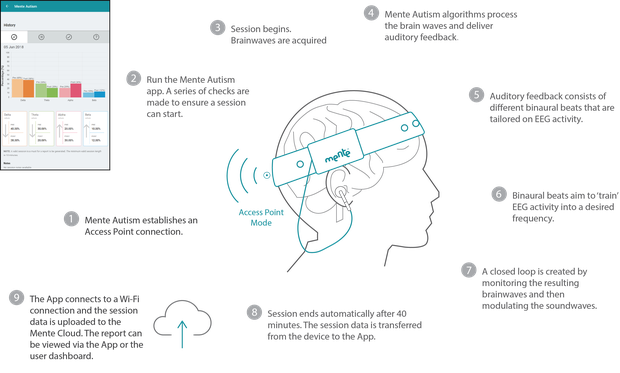
Each child will hear different sounds when they use the device, but positive effects are typically seen after four to eight weeks of 40 minute daily sessions, with the technology recommended for children aged between three to 12 years.
The data is uploaded to the Mente Cloud server, which generates a report measuring the session's progress and the information can be viewed online or within the app.
A clinical US trial testing the efficacy of the Mente Autism device has produced significant positive changes for children with ASD. The full results were published by medical journal, Frontiers in Neurology.
The study was administered by the Carrick Institute, and was a randomised-controlled, double-blind investigation that compared Mente Autism with a control (or sham) device over a 12-week period.
The Carrick Institute recruited 83 participants, after determining that at least 32 participants were required and allowing for a reasonable level of drop-outs. At the trial’s conclusion, 34 participants (17 active, 17 control/placebo) remained, ranging in age from five to 17 years old.
Notably, while some subjects were ultimately not included (for example, due to inability or unwillingness to return for the post-trial evaluations), there were no drop-outs due to problems of tolerance with the Mente device or treatment.
Using Mente Autism, the trial found the device can lead to significant changes in brain activity (qEEG), sensorimotor behavior (posturography), and behavior (standardised questionnaires) in children with ASD.
Researchers saw a range of positive results, including improvement in social skills and communication, as well as improved balance.
“When we first designed the study, Mente Autism was of interest as it is a novel tool that takes clinical neurofeedback, an established non-invasive biofeedback approach for the treatment of autism, and makes this really accessible as a treatment option for parents of children with autism,” said Dr Frederick Carrick, principal investigator for the US clinical trial and Professor in Neurology at Cambridge University.
Carrick highlighted the positive feedback and gratitude received from parents in the study’s active group, who noticed improvements in communication and social skills in their children.
Carrick appeared on 6PR radio in Perth earlier this week to discuss how the Mente Autism headband works, as well as its implications:

The Mente Autism device already has European CE Marking and TGA Registration, thereby enabling sales in Europe, the Middle East and Australia.
Neurotech recently completed the first shipments of an improved version of the Mente Autism device, now in its third iteration. The company is also looking to obtain US FDA clearance for the device, with the US clinical trial providing compelling evidence to accompany submission.
Given the limitations of traditional neurofeedback, including considerable cost, time and effort from parents and their children to attend sessions in clinical environments, the positive outcomes of the US clinical study — alongside an expanding stockpile of positive testimonials for parents — suggest that Mente Autism is uniquely placed as a home-based neurofeedback tool.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.






