MMJ takes major step forward on phase 2 trials
Published 16-AUG-2016 15:03 P.M.
|
2 minute read
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
MMJ PhytoTech (ASX:MMJ) has taken a major step forward on its journey to becoming a pharmaceutical player in the epilepsy space – confirming phase 2 trials for Q4.
It told its shareholders yesterday that following on from successful phase 1 trials in the first quarter this year that it was now in the process of designing phase 2 trials for its Gelpell PTL101 capsule, with the trial to be run out of Israel.
The phase 1 trial was a success for MMJ, where it successfully demonstrated that it had a better cannabidiol (CBD) and THC absorption rates than market-leading spray Sativex, from GW Pharmaceuticals.
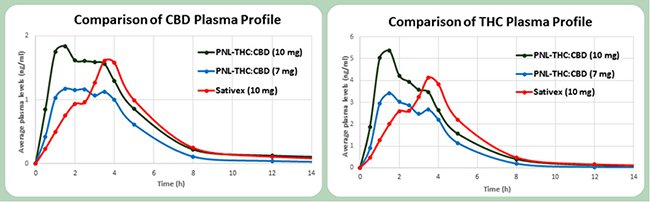
Results of the phase 1 clinical trial
This means the active ingredients are able to work faster than the market leading brand, although there is still water to go under the bridge still.
MMJ will be hoping the phase 2 trials confirm the product’s efficacy against other solutions on the market, with MMJ managing director Andreas Gedeon saying MMJ was truly unique in the space.
“While many other companies try and fail in attempting to reach this stage, our ability to compete with a large pharmaceutical player like GW results from the synergies of our vertically integrated global supply chain,” he said.
MMJ still has a long way to go before being matched to GW’s share price or market cap, so while competitive on product you should still take a cautious approach to making an investment decision in this company and seek professional financial advice.
MMJ operates several arms with a cultivation and extraction operation in Canada, a product development arm in Switzerland, and a R&D unit in Israel.
A phase 2 trial has the potential, the company said, to act “as a key catalyst” towards the commercial development of the PTL101 prescription drug.
The development of another drug would also be beneficial to those suffering from intractable epilepsy – with 100,000 children in the US alone suffering from the treatment-resistant category of the disease.
It is estimated that treatment remains ineffective for as many as 30% of sufferers, due to drugs failing to control the frequency of seizures or patients not being able to manage the side-effects of the drugs.
In other MMJ news, it told its shareholders that it in the final stretch of designing a phase 2 trial on its PTL201 drug, aimed at managing spasticity associated with multiple sclerosis.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.






