MMJ oral pill passes Phase 1 Clinical Trials
Published 11-FEB-2016 10:11 A.M.
|
3 minute read
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
MMJ Phytotech (ASX:MMJ) has taken its first steps towards its end-goal of filing a New Drug Application (NDA) with the US Food and Drug Administration (FDA) in order to grow and supply medicinal cannabis. MMJ is the first medical grade cannabis company to be listed on the ASX, operating a ‘farm to pharma’ strategy that could see the company bloom into a pharmaceutical company with full supply chain integration.
Looming changes in national legislation within Australia are likely make growing and selling medical cannabis less restrictive therefore paving the way for MMJ to commence selling medical cannabis products as soon as legislation allows.
As part of a regulatory filing with the ASX, MMJ reports that its trials “indicate that MMJ’s formulations are the same or even higher performance level when compared to GW Pharmaceutical’s product”.
The trial involved administering dosages of medicinal cannabis to 15 healthy volunteers of two oral THC:CBD formulations in comparison to Sativex, the leading oromucosal medicinal cannabis spray from GW Pharmaceuticals.
A Phase 1 clinical trial of MMJ’s oral capsules was undertaken at the Souravsky Medical Clinical Research Center in Israel, through a “single-centre, multi-arm, randomised, crossover study” assessing the safety, tolerability and pharmacokinetics of MMJ’s product.
The results in detail
The results of MMJ’s Phase 1 Trials are extremely positive with consistent results among all 14 test subjects that completed the trial.
The Trial yielded the following results:
- Demonstrable safety and tolerability profile with no significant side effects;
- Higher bioavailability of active compounds in comparison to GW Pharmaceuticals oromucosal spray – Sativex;
- Very rapid onset; and
- 8 hours exposure time in the blood
A comparison of plasma concentration over time between MMJ’s CBD/THC capsule and GW’s nasal spray indicates that MMJ’s capsules have a quicker uptake time and longer-lasting effects within the patient.
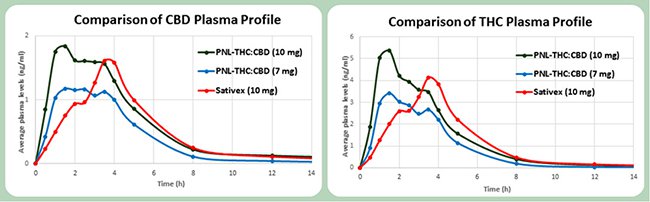
Having succeeded at Phase 1, MMJ now plans to move onto Phase 2 “in the second half of 2016”. Phase 2 will assess the efficacy of oral capsules in treating pain and spasticity amongst multiple sclerosis sufferers, with results expected in 2017.

Sourasky Medical Center in Tel-Aviv
MMJ has also announced an extension of its license agreement with Yissum, the commercial arm of Hebrew University of Jerusalem. According to an ASX announcement, MMJ’s extension of the exclusive licensing agreement with Yissum will “include the addition of two new US provisional patent applications” thereby improving MMJ’s IP Portfolio.
The first additional patent comprises active combinations of THC and CBD in a dispersible concentrate. The second patent comprises dry, rapid water dispersible nano-particles containing cannabinoids incorporated into a biodegradable polymer. MMJ says this patent’s “unique formulation offers high yield, extended release, high bio-availability and water solubility cannabinoids”, that “can be delivered in oral, inject-able, topical, inhaled and ear/eye drop formulations”.
MMJ Growing in Canada
ASX-listed MMJ has growing operations currently undergoing regulatory checks with a full growing license expected to be awarded within weeks.
The Duncan facility close to Vancouver, Canada will produce an estimated 1000kg of high grade medicinal cannabis per year, once fully up and running.
While MMJ progresses its growing operations under Canadian subsidiary United Greeneries, its Israel-based unit, Phytotech Theraputics is progressing with clinical trials of MMJ’s CBD-based oral capsule, designed for treating multiple sclerosis.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.






