Immuron generates strong Travelan sales growth
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
Microbiome biopharmaceutical group, Immuron Limited (ASX:IMC), continues to deliver strong fiscal 2018 sales growth in relation to its over-the-counter gastrointestinal and digestive health supplement Travelan®.
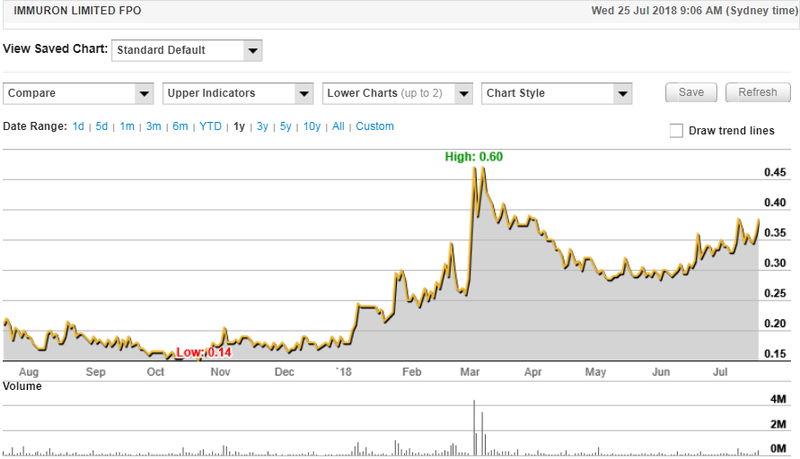
Global Immuron Ltd sales revenue reached $2 million, maintaining its upward trend with sales up more than 45 per cent compared with fiscal 2017.
In Australia, Travelan® sales increased 19 per cent, while substantial growth was generated overseas.
US Travelan® sales continue to exceed expectations growing to $765k which represents a 112 per cent increase over FY17.

It should be noted that continued sales growth at this rate is speculative, so investors should seek professional financial advice for further information if considering this stock for their portfolio.
Immuron and the Travelan story
From a broader perspective, Immuron is an Australian company focused on developing and commercializing orally delivered targeted polyclonal antibodies for the treatment of inflammatory mediated and infectious diseases.
The company currently markets and sells Travelan® for the prevention of travelers’ diarrhea and its lead clinical candidate, IMM-124E, is in Phase II clinical trials for Non-Alcoholic Steatohepatitis (NASH), Severe Alcoholic Hepatitis (SAH) and Pediatric Non-alcoholic Fatty Liver Disease (NAFLD).
Immuron’s second clinical stage asset, IMM-529, is targeting Clostridium difficile Infections (CDI).
The company’s shares have traded strongly in the last month on the back of a patented grant for its Non-alcoholic steatohepatitis (NASH) treatment and successful progress with a research collaboration arrangement with the US Department of Defence.
The past performance of this product is not and should not be taken as an indication of future performance. Caution should be exercised in assessing past performance. This product, like all other financial products, is subject to market forces and unpredictable events that may adversely affect future performance.
Strong sales momentum heading into fiscal 2018
Importantly, the strong sales of Travelan® in the first three quarters continued into the June quarter, perhaps an indicator that momentum should continue in fiscal 2019.
US Travelan® sales were assisted by exceptional fourth quarter sales of $265,000 which is a 47 per cent increase on the same period last year.
In Australia, fourth quarter Travelan® sales of $350,000 represented a 53 per cent increase compared with the previous corresponding period.
Attributing a significant part of the success to the company’s marketing strategy, Dr Jerry Kanellos, chief executive of Immuron said, “Our focused marketing efforts demonstrated by the team are helping to build awareness and educate both consumers and travel medicine clinicians on the benefits of Travelan®.
“We are confident that our sales growth will continue as our marketing strategy builds upon this exposure”.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.