BD1 gets Australian patent for cancer-related tech
Published 07-AUG-2017 14:54 P.M.
|
2 minute read
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
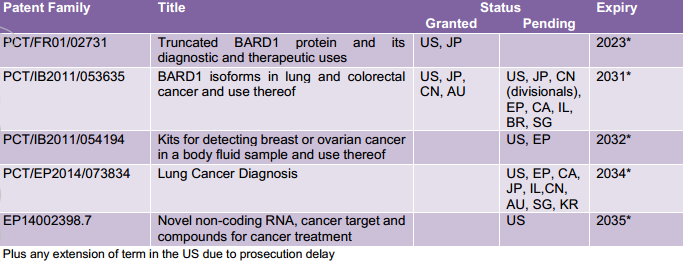
BARD1 Life Sciences (ASX: BD1), a company focused on developing and commercialising non-invasive diagnostic tests for early detection of cancer, has gained patent approval from IP Australia for its “BARD1 isoforms in lung and colorectal cancer and use thereof”.
Chief Executive, Dr Learnne Hinch highlighted that this core patent family now has four granted patents in the US, Japan and China, as well as Australia.
This patent family protects the sequence of various BARD1 isoforms specific to lung and colorectal cancer, a method for detecting the presence of the specific BARD1 isoforms, and a method for treating and/or preventing lung cancer and colorectal cancer.
BD1’s patent portfolio as highlighted below includes five patent families covering various BARD1 DNA and protein sequences, methods of diagnosis and treatment, and use in multiple cancers.
The group’s lung cancer test is currently in development for the early detection of lung cancer, the fifth most common cancer and the leading cause of cancer deaths in Australia.
It should be noted, however, that this is an early stage biotech company and success is no guarantee. Investors should seek professional financial advice before making an investment.
The Australian Institute of Health and Welfare (AIHW) estimates that there will be 12,434 new cases of lung cancer diagnosed and 9,021 deaths from lung cancer in Australia in 2017. The latter represents 18.9% of all projected cancer deaths.
Hinch highlighted the fact that lung cancer is often diagnosed at a later stage after it has spread to other parts of the body, resulting in a 16% chance of survival after five years in Australia.
Early detection of lung cancer has the potential to save lives by enabling early treatment when it is potentially curable, but there is currently no approved blood test available for early detection of lung cancer in people without symptoms.
BD1’s technology uses novel tumour markers and a proprietary algorithm. The company also has a pipeline of technologies with similar goals such as the Ovarian Cancer Test which is aimed at the early detection of ovarian cancer, and the company also has high-value diagnostic and therapeutic projects at research stages for multiple cancers.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.



