BARD1 announces positive results from ovarian cancer test
Published 10-JAN-2018 16:04 P.M.
|
3 minute read
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
BARD1 Life Sciences Limited (ASX:BD1), a biotechnology company developing non-invasive cancer diagnostics, yesterday informed the market it has received positive results from its OC-400 Study that showed BARD1-Ovarian achieved high accuracy for detection of ovarian cancer.
The encouraging results included an average AUC (area under the curve) of 0.92 in the training sets, and an average AUC of 0.88, 82 per cent sensitivity and 79 per cent specificity in the test sets.
BARD1 has now successfully completed the retrospective, case-control, OC-400 Study which was intended to evaluate the accuracy of the multi-analyte BARD1-Ovarian test to detect ovarian cancer in 400 female bio-banked samples of ovarian cancer and aged-matched healthy controls.
The specific objective of the company’s latest study was to further improve the BARD1 panel and algorithm used in the BARD1-Ovarian, a test which the company hopes will be used for early detection of ovarian cancer. Further, the study assessed the accuracy of the test using the proof-of-concept (POC) method on a panel of 20 peptides (analytes).
All in all, the study determined that BARD1-Ovarian accurately detected ovarian cancer and thereby provides BARD1 with further rationale for developing it into a commercial early detection test.
It should be noted that BARD1 is in its early stages here and investors should seek professional financial advice if considering this stock for their portfolio.
Data analysis of the results produced a model with:
- an average AUC of 0.92, 90% sensitivity and 85% specificity in the training sets, and
- an average AUC of 0.88, 82% sensitivity and 79% specificity in the cross-validation test sets.
The table below provides a summary of the results of BARD1-Ovarian in the OC-400 Study:
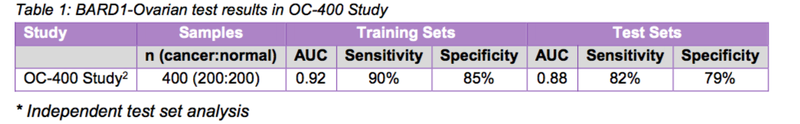
Source: BARD1 Life Sciences
These findings support what the company determined via previous studies — that BARD1-Ovarian could detect ovarian cancer “with high sensitivity and specificity”.
Considering ovarian cancer is the eighth most common cancer and the sixth most common cause of cancer death affecting women in Australia, this is a significant finding, particularly for a small-cap biotech company such as BARD1.
In further positive news, the most recent test results were achieved using less analytes, reducing the complexity and cost of the BARD1-Ovarian test — which could bode well for it when compared with the CA125 blood test routinely used as a diagnostic aid for ovarian cancer.
The study concluded that BARD1-Ovarian accurately discriminated ovarian cancer from healthy controls using the POC method and a 20-analyte panel, could detect all subtypes and stages of ovarian cancer, and confirmed the potential of the research-grade test to be further developed into a commercial test with expected high sensitivity and specificity for early detection of ovarian cancer.
BARD1 Executive Director and CSO, Dr Irmgard Irminger-Finger, said “this study showed that using the POC method, BARD1-Ovarian achieved better sensitivity and specificity in a sample set from multiple- sites with a reduced number of analytes than previously reported in the OC-300 Study.”
“The positive results achieved in this OC-400 Study confirmed the potential of BARD1-Ovarian to accurately detect ovarian cancer with high sensitivity and specificity,” BARD1 CEO Dr Leearne Hinch said.
“The reduced analyte panel is an important step forward in the development of an accurate and affordable BARD1-Ovarian test for early detection of ovarian cancer.”
BARD1 intends to advance the development of BARD1-Ovarian, with the potential for outsourcing further assay development to a contract development organisation, in readiness to build it into a commercial test.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.



