ASX pot-stock MGC Pharma mixes up first CannEpil™ batch
Published 29-MAR-2018 11:52 A.M.
|
3 minute read
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
MGC Pharmaceuticals (ASX:MXC) this morning revealed that its European medicinal cannabis manufacturing facility is today producing the first batch of CannEpilTM, following the receipt of its interim Good Manufacturing Practice (GMP) certification.
This sizeable milestone kicks off following delivery of pharmaceutical-grade THC compound to the company’s manufacturing facility and approval from the European authorities.
Analysis of the first batch will commence shortly to confirm that GMP production protocols have been followed. Critically, this will be the final step in the process before full certification is granted, after which the facility will be one of the most advanced in Europe for pharmaceutical-grade medical cannabis production.
Once granted, MXC will be able to begin full-scale commercial production of CannEpil, and to produce additional pharmaceutical-grade medical cannabis products for use by the company in clinical studies, research pipelines and additional medical products licensed for distribution.
CannEpil is MGC Pharma’s first pharmaceutical-grade medical cannabis product, targeting drug-resistant forms of epilepsy (also known as refractory epilepsy), which accounts for around 30 per cent of the 240,000 people diagnosed with epilepsy in Australia each year.
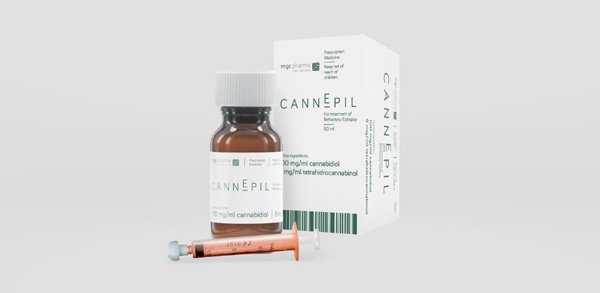
Once distribution of CannEpil begins, moreover, it is set to generate significant revenues for MXC, expected to be around $1 million per year from its first full year of distribution. Notably, this is from less than 100 patients already registered through MXC’s relationship with Epilepsy Action Australia (EAA).
CannEpil be distributed in Australia under MGC Pharma’s agreement with specialist Australian pharmaceutical distributor, HL Pharma.
It’s worth noting here that this is an early stage play and investors should seek professional financial advice if considering this company for their portfolio.
MXC co-founder and CEO, Roby Zomer, said: “This is an exciting final step for MGC Pharma as we focus on completing full GMP certification of our manufacturing facility, allowing us to produce CannEpil in Europe and focus on significantly progressing our medical research and development of our pharmaceutical products pipeline.”
“The production of our first CannEpil batch is a milestone, and leaves us well-positioned to achieve certification and commence full-scale commercial production in the next few months.”
This most recent development — a sizeable landmark for the $103.4 million-capped biotech junior — has put MXC on a considerable high. The company’s share price has surged on the back of this morning’s announcement, currently sitting at 8.9 cents.
Past performance is not necessarily indicative of future results. As part of the due diligence process, investors must consider all factors over and above the past performance of the product. Investors should not engage with a product solely on it past performance.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.






