Imugene to demonstrate the benefits of combination tumor treatment
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
Imugene Limited (ASX:IMU) has provided a promising update on the preliminary clinical development plan (CDP) for the proposed exclusive license of the patents covering the CF33 oncolytic virus technology, which is subject to shareholders’ approval at an Extraordinary Meeting of Shareholders to be held on 18 November, 2019.
The CF33 oncolytic virus (OV) was developed in the laboratory of Professor Yuman Fong, an internationally recognised surgeon and scientist at City of Hope, a California-based world-renowned independent research and treatment centre for cancer, diabetes and other life-threatening diseases.
CF33 has been developed in two different constructs.
One version of the OV is “armed” with an immune checkpoint inhibitor inserted in the virus, which is known as CheckVacc.
The other is an unarmed construct, known as Vaxinia, and Imugene plans to conduct two separate Phase 1 clinical trials in 2020 to test a CheckVacc construct and a Vaxinia construct of the OV.
Early positive signs in breast cancer studies
Pre-clinical studies conducted at City of Hope by Professor Fong have shown encouraging results in triple negative breast cancer (TNBC) when CF33 is combined with an immune checkpoint inhibitor (ICI), specifically a PD-L1 inhibitor to yield CheckVacc.
TNBC is an aggressive subtype of breast cancer affecting 20% of breast cancer patients with poor prognosis upon diagnosis of metastases, largely due to lack of effective targeted therapy.
Immune check point inhibitors have shown efficacy in TNBC1s.
In March 2019 the FDA approved Genentech’s, member of the Roche group, PD-L1 ICI Atezolumumab (Tecentriq) for TNBC.
Immune checkpoint blockade has shown great promise as novel class of drugs to treat certain types of advanced cancers in the last five years.
However, only a limited percentage of cancer patients achieve objective clinical responses through ICI treatments, leaving significant room for improvement partly due to complicated regulatory circuits of immune functions in cancer.
Professor Fong’s pre-clinical studies have 1shown that CF33/ICI combinatorial therapy may be applicable to a much wider population of cancer patients, and there is increasing commentary in medical journals suggesting that combination therapy offers the best of both worlds.
The Phase 1 trial commencing in 2020 will be an open-label, dose-escalating, non-randomized, single-centre phase 1 study of CheckVacc administered intratumorally in patients with metastatic TNBC with injectable metastatic lesions.
The purpose of the study will be to evaluate the safety and initial evidence of efficacy of the CF33-antiPDL1 combination oncolytic virus against TNBC.
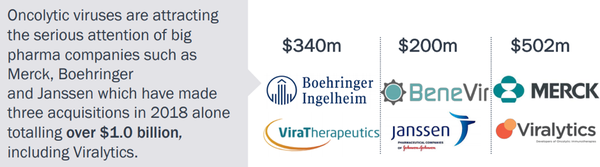
CF33 progress bodes well for Vaxinia
Imugene referenced commentary from numerous high-impact medical journals in noting that impressive activity of the CF33 oncolytic virus Vaxinia has been demonstrated in multiple solid tumour types in validated invivo models of pancreatic, colorectal, lung, TNBC and colon cancers.
Importantly, Vaxinia outperforms the industry leading OV’s from Amgen and Genelux.
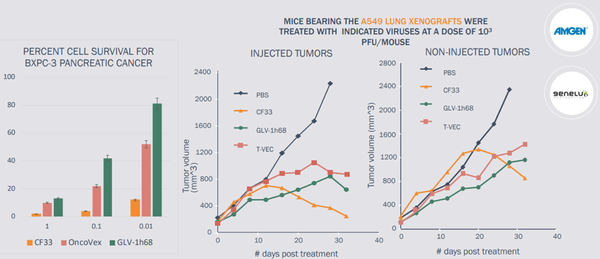
Vaxinia is more potent than its competitors and a strong advantage is the level of dosing required, at least in preclinical animal models, is much lower.
The Phase 1 MAST (Mixed Advanced Solid Tumors) trial commencing in 2020 will be an FDA IND Imugene sponsored open-label, dose-escalating, non-randomised, multi-centre (including Australian hospitals) phase 1 study of Vaxinia administered intratumorally or intravenously in patients with solid tumors (lung, TNBC, melanoma, bladder, GI).
The purpose of the study will be to evaluate the safety and initial evidence of efficacy of the CF33 Vaxinia oncolytic virus against solid tumors.

General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.