Proteomics awarded vital CE Mark registration for PromarkerD
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
Proteomics International Laboratories Ltd (ASX:PIQ), a pioneer in predictive diagnostics, has secured CE Mark registration for its PromarkerD Immunoassay kit as an IVD medical device (in vitro diagnostic).
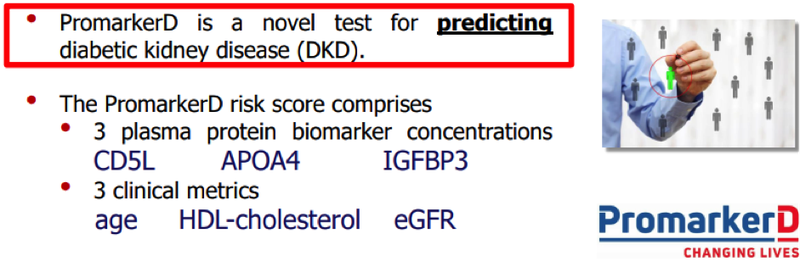
PromarkerD (IA) forms part of the PromarkerD test system which is the only test available in the Europe Union for predicting the onset of diabetic kidney disease (DKD).
The PromarkerD Immunoassay is the high-throughput version of the already CE Marked PromarkerD (MS) Mass Spectrometry test system.
This will allow a greater number of prospective laboratories to process much higher numbers of samples at a more cost effective rate.
This is a key market for Proteomics as the company’s business model is centred on the commercialisation of its world-leading test for diabetic kidney disease, PromarkerD.
The group offsets the cash burn from research and development and product development through provision of specialist analytical services, whilst using its proprietary Promarker technology platform to create a pipeline of novel diagnostic tests.
Low-cost test provides early warning
PromarkerD was registered for use in the EU as part of the company’s program of regulatory and reimbursement registrations.
The PromarkerD test system assesses the risk of DKD in patients with type 2 diabetes.
The World Health Organisation estimates the European region is home to 60 million adults with diabetes, and currently one in three have chronic kidney disease.
Chronic kidney disease is one of the major complications arising from diabetes and if unchecked can lead to dialysis or kidney transplant.
PromarkerD is a simple, low-cost blood test that uses a unique protein ‘fingerprint’ to detect the onset of disease up to four years before clinical symptoms appear.
The early detection and prevention or treatment of DKD is a major focus of both global pharmaceutical companies and government health departments.
CE Mark registration provides assurance to licensing partners
The CE Mark is a significant commercialisation step for Proteomics International, providing assurance to potential licensing partners and consumers that the product has been developed and manufactured to meet EU safety, health and environmental protection requirements.
Dr Richard Lipscombe, managing director of Proteomics International highlighted the benefits of high-volume testing in saying, "The CE Mark is another important milestone for PromarkerD as we move forward with new deals in the region.
‘’With CE Mark the immunoassay offers a higher-throughput option for testing laboratories who want access to our ground-breaking test for predicting diabetic kidney disease — one that could enable earlier therapeutic intervention to minimise the effect of this crippling disease."
Achieving CE Mark registration for the PromarkerD (IA) provides a strong foundation for PromarkerD to receive US FDA approval.
Proteomics International intends to lodge the US FDA application in mid-year 2020.
Given PromarkerD can aid clinical decision making by identifying at-risk individuals for earlier therapeutic intervention to help to minimise the effect of the disease, potentially saving healthcare systems billions of dollars there are definite incentives for other countries to support its introduction.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

