OncoSil™ receives CE Mark approval
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
OncoSil Medical Ltd (ASX:OSL) has received CE Marking approval from the British Standards Institute (BSI) for its OncoSilTM device used in the treatment of locally advanced pancreatic cancer (LAPC) in combination with chemotherapy.
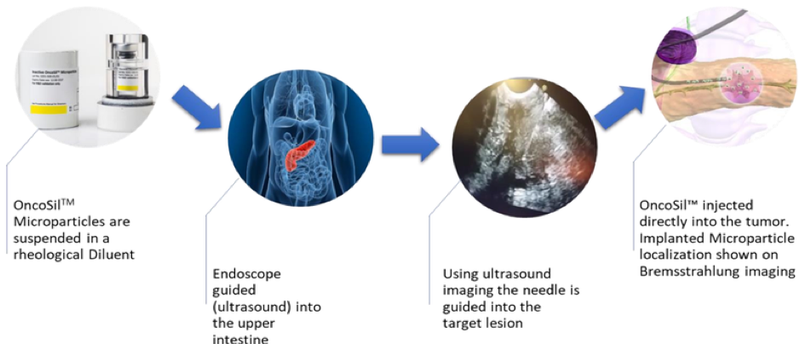
OncoSilTM is a first in class medical device comprising microparticles containing phosphorus-32 (P-32), a pure beta-emitter radioisotope, implanted directly into a patient’s pancreatic tumour via endoscopic ultrasound guidance.
This first approval is a major milestone for OncoSil and allows for the OncoSilTM device to now be marketed and sold within the European Union (EU) and the UK.
Noting ongoing Brexit discussion, the CE Mark granted to OncoSil Medical provides market authorisation also to the UK, as well as the EU.
This is an extremely important, commercially significant development for the company as CE Marking certification is a validation of the OncoSilTM device and its clinical performance.
Clinical studies by Oncosil have demonstrated a median overall survival of 16.1 months in patients treated with OncoSilTM plus chemotherapy (CT) almost double the accepted median overall survival in patients with unresectable pancreatic cancer.
OncoSil chief executive Daniel Kenny said, “Designation of the OncoSilTM device as a breakthrough device is a validation of our platform technology which can be used to treat multiple solid tumour types such as liver, biliary duct and of course pancreatic cancer.
“Having secured CE Marking approval, our focus is now on multiple registration filings in jurisdictions which recognise CE Marking certification.
‘’It is worth noting that this activity is unaffected by the COVID19 pandemic,” added Mr Kenny.
However, the COVID-19 pandemic will impact launch preparedness and delay OncoSil’s European launch as management expects disruptions due to limited hospital access in the coming months for new site initiation and training as well as shipping and logistical disruption.
Breakthrough device designation is recognition of importance
In addition to CE Marking approval, the OncoSilTM device has now been officially classified as a “breakthrough device” as defined under EU Medical Device guidance.
In the EU a “breakthrough device” is defined as one that delivers clinical benefit to patients for unmet medical needs which are life threatening, and for which current medical alternatives are insufficient or carry significant risks.
The OncoSilTM device is now officially designated as a breakthrough device in the EU and the UK, and this come shortly after receiving the same designation in the US.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.