Noxopharm receives FDA green light to progress towards Veyonda® trial
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
Shares in Noxopharm Ltd (ASX:NOX) traded more than 40% higher on Tuesday afternoon after the company received advice from the US Food and Drug Administration (FDA) that it should lodge a pre-Investigational New Drug (pre-IND) submission for a clinical trial of Veyonda® in patients with SARS-CoV-2 (COVID-19) infection.
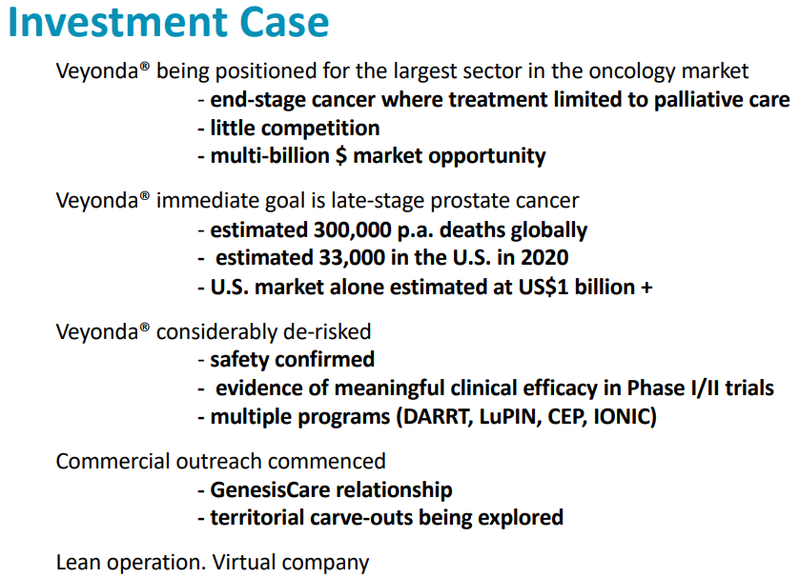
Noxopharm is a clinical-stage Australian drug development company with its primary focus being the development of Veyonda®.
The submission is based on a response to a package submitted to the FDA summarising the rationale for conducting a clinical trial with an inhibitor of cGAS-STING signaling.
The urgency of the situation means that if the pre-IND is evaluated positively by the FDA, it can be converted into a fully expedited IND approval.
The conversion of a pre-IND into a full IND is a new option offered to high priority COVID-submissions, significantly reducing the time and complexity of the FDA review process.
Veyonda® has a mechanism of action that Noxopharm believes marks it as a prospective treatment of septic shock in COVID-19 patients, a condition associated with inflammatory and clotting problems and believed to be contributing to multi-organ failure and death in COVID-19 patients.
Idronoxil a potent inhibitor of cGAS-STING signalling pathway
Pre-clinical research conducted by the Hudson Institute of Medical Research has shown that one of the anti-cancer mechanisms of action of idronoxil (the active ingredient in Veyonda®) is potent inhibition of the cGAS-STING signalling pathway.
The cGAS-STING signalling pathway is responsible for alerting the body’s immune system to the presence of an invading virus by triggering the production of cytokines.
This pathway is critically important in generating an immune response that contributes to the great majority of COVID-19 patients recovering uneventfully.
However, in a small proportion of patients who develop breathing problems leading to low oxygen levels, tissue damage in major organs triggers a second and excessive wave of cGAS-STING signalling, resulting in a so-called ‘cytokine storm’, amplifying existing tissue damage and inducing blood clotting problems.
A number of COVID-19 clinical trials are being conducted with drugs inhibiting individual components of the ‘cytokine storm’ such as IL-6 and TNF -alpha.
The basis of the Noxopharm submission to the FDA is that by inhibiting the cGAS-STING signalling pathway, Veyonda® offers a special opportunity to block a broader range of cytokines at their source, potentially preventing it altogether or reducing the severity of septic shock and the number of patients dying from it.
The proposed study involves dose-response, dose confirmation and dose expansion with the latter involving a comparison of Veyonda® with current standard of care treatment.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.



