Invion: Making a dynamic play in cancer treatment
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
Drug delivery company Invion Limited (ASX:IVX) is leading the way in developing PhotosoftTM technology for the treatment of a range of cancers. Managing director and CEO, Dr Greg Collier spoke with Finfeed’s Jonathan Jackson about the progress this life sciences company is making in cancer treatment, it’s partnership with the Cho Group and why Invion should be on investors’ radars.
“I’ve been a CEO for over 20 years,” says Invion CEO Dr Greg Collier. “Normally I am out talking to investors about capital raises and clinical trials, but my biggest message as CEO of Invion is we have a solid clinical program, an excellent management team and we do not need to raise capital. That puts us in a unique position as a life sciences company working in cancer treatment.”
The message is clear from Dr Collier, who knows a thing or two about what it takes to run a successful company in the life sciences sector. He was previously the CEO of ChemGenex, which was acquired by Cephalon for $200+ million in 2011. Teva (NASDAQ:TEVA), in turn, acquired Cephalon, for $6.8 billion.
Dr Collier, who is a past recipient of the Roche Award for Excellence, may not be thinking in terms of hundred million dollar acquisitions ... just yet... but he is certainly buoyant about the work Invion is doing in what could be lucrative markets for the company.
The Basal Cell Carcinoma (BCC) and Actinic Keratosis (AK) markets, in Australia alone, are estimated to be worth $703 million and growing. The global BCC market was valued at approximately US$5.3 billion in 2017 and is expected to generate revenue of around US$10.0 billion by end of 2025. Market growth is expected at a CAGR of around 9% between 2018 and 2024.
The global AK market was valued at US$6.5 billion in 2015 and is expected to reach US$8.9 billion by 2022. Market growth is expected at a CAGR of around 4.35%.
So why is Dr Collier buoyant about Invion’s impact in these markets? It’s all down to the technology.
What is photodynamic therapy?
Photodynamic therapy (PDT) is a treatment that uses a drug, called a photosensitizer or photosensitizing agent, and a particular type of light. When photosensitizers are exposed to a specific wavelength of light, they produce a form of oxygen that kills nearby cells.
However, there were problems with the original treatments with regard to this form of therapy.
“The old PTD therapies had problems because they were fat soluble and tended to stay in the body much longer,” Dr Collier says. “People couldn’t go out into the sun for three to four months and light didn’t penetrate far into the cancer cell. Recurrence was also high.”
Invion’s treatment, known as PhotosoftTM, is an extension of this therapy, but looks to have significant upside from what has been on offer in the past.
Named as one of the best performing small caps of 2018, this $95 million capped company’s Photosoft technology gives it a unique place amongst its biotech and life sciences peers.
How the technology works
Photosoft is a photosynthesiser made from chlorophyll, which is activated at certain light sensitivity levels and produces “cyto-reactive oxygen”, which can kill malignant cancer cells.
“As it is chlorophyll based and soluble, there are no side effects and it doesn’t accumulate in the body,” Dr Collier says.
He goes onto to say that the Photosoft treatment can treat all cancer cells. Late last year, Invion informed the market that a new formulation of its Photosoft technology was 15 times more effective at killing ovarian cancer cells than the previous iteration.
Photosoft can be taken intravenously or topically, with IVX-P02 currently the star player.
IVX-P02 is 15 times more effective in killing cancer cells in in vitro tests against ovarian cancer, compared to the previous iteration – PhotosoftTM Oral.
IVX-PO2 has also shown enhanced cytotoxicity (toxic to cells) in vitro compared to existing, commercially available products Talaporfin and Temoporfin.
These are the benefits of Photosoft technology:
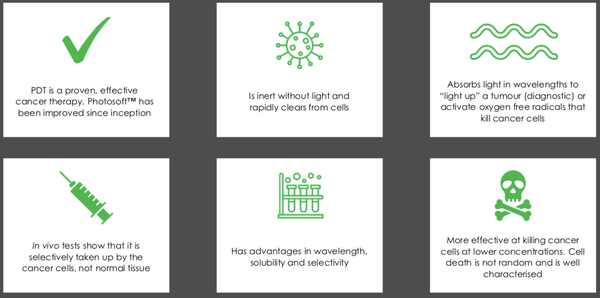
Invion’s PDT topical product IVX-SKIN treats skin cancer, the most common type of cancer affecting over 50 million people worldwide.
IVX-SKIN treats:
- Basal Cell Cancer (BCC)
- Actinic Keratosis (pre-cancer)
- Squamous Cell Cancer (SCC)
The product is positioned as a low pain treatment that is better than surgery and more effective than commercially available product Metvix.
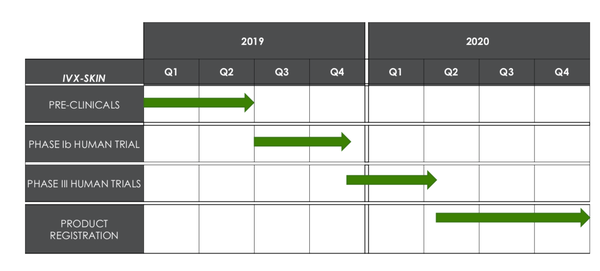
Invion is currently undertaking its Skin Cancer Phase Ib trial, which will be followed by a Phase III trial to prove the above hypothesis in the December quarter.
It will continue to be a busy year for Invion.
Here’s what to expect:
Who’s backing the trials?
Cho Group Limited is backing the trials. It funds and develops advanced technology humanitarian and environmental projects, combining an ethical business model with highly profitable businesses.
The Group doesn’t align itself with companies lightly and it sees a lot of potential in Invion, so its strategic alliance with the ASX listed company is something of a coup.
The Cho Group is the inventor and owner of Photosoft technology and appointed Invion as exclusive distributor and licensee of the Photosoft technology in Australia and New Zealand for the treatment of cancers.
Invion was engaged to conduct the clinical development of Photosoft globally, initially targeting prostate cancer in Australian-run clinical trials.
That purview has now greatly expanded, with both companies expected to benefit.
“Our agreement with Cho means we can operate in a non-dilutive manner, without having to raise capital,” Dr Collier explains.
“We have guaranteed funding for all R&D.”
The Hudson line
Invion’s partnership with the Hudson Institute has also proved to be of great benefit.
Back in March 2018, Hudson entered into a R&D Alliance Agreement with Invion. Its initial focus was on ovarian cancer, with Hudson providing the research facilities and expertise required to undertake individual Invion-sponsored research projects, including Photosoft.
In the following interview, Hudson’s cancer expert Dr Andrew Stephens discusses the collaboration:
Embed: YouTube video: https://www.youtube.com/watch?v=dbvDMAr4DXQ
The partnership has developed significantly since it was first formed. Invion has moved into its trial phases and Hudson has been with it every step of the way.
Dr Stephens is now part of Invion’s Scientific Advisory Board, advising on Invion’s multiple Photosoft projects.
Invion has been busy strengthening its board this year, having announced three further appointments including Dr Lynda Spelman (skin cancer), Assoc. Professor Louis Irving (lung cancer), and Assoc. Professor Nathan Lawrentschuk (urological cancer including prostate cancer).
“They bring a wealth of academic and clinical experience and have previously interacted positively with industry over a number of years,” Dr Collier said.
“The appointments will advance our clinical planning and ensure that Invion’s drug development is optimised for each different cancer type.”
A big second half
With several trials now in play and further trials to begin, Invion has a lot on its plate.
Trials have already proved to be successful, but for Dr Collier the really good news for shareholders is yet to come.
“The real success drivers moving forward are the clinical outputs, the efficacy of skin cancer treatment with hopefully no pain and no associated scarring and the effective treatment of carcinoma patients.”
These drivers should play out in the coming six months.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

