Antisense appoints experienced biotech executive
Published 28-FEB-2020 11:08 A.M.
|
3 minute read
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
Antisense Therapeutics Limited (ASX:ANP) announced on Thursday that it had appointed Gil Price M.D. as Consultant Medical Director.
Dr. Price is a clinical physician trained in internal medicine with a long-standing focus in drug development, adverse drug reactions, drug utilization and regulation.
He is an experienced biotech executive and entrepreneur with a depth of expertise across clinical asset investment strategy, evaluation, financing and execution.
Gil’s experience should be valuable in assisting Antisense Therapeutics given its position as an Australian publicly listed biopharmaceutical company, developing and commercialising antisense pharmaceuticals for large unmet markets.
The products are in-licensed from Ionis Pharmaceuticals Inc. (NASDAQ:IONS), world leaders in antisense drug development and commercialisation.
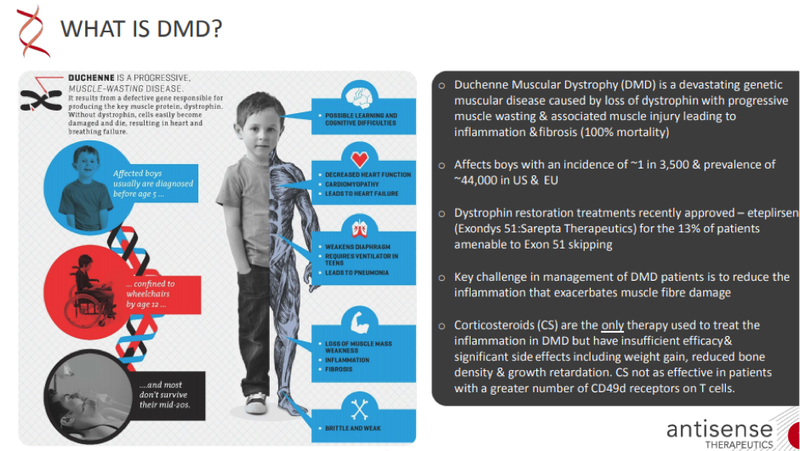
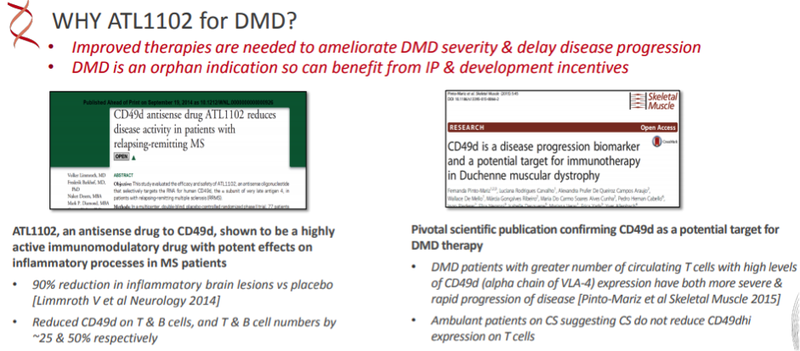
As a backdrop, one of its key products, ATL1102 (injection) has successfully completed a Phase II efficacy and safety trial, significantly reducing the number of brain lesions in patients with relapsing-remitting multiple sclerosis (RRMS).
The company’s ATL1103 drug designed to block GHr production successfully reduced blood IGF-I levels in Phase II clinical trials in patients with the growth disorder acromegaly.
Antisense Therapeutics is also conducting a Phase II clinical trial of ATL1102 in DMD patients at the Royal Childrens Hospital, Melbourne.
Price transforms Sarepta Therapeutics to multi-billion dollar company
Over a lengthy period, Price has served on multiple boards of public, private and not-for-profit entities.
From 2007 to 2016, he was a non-executive director of Sarepta Therapeutics, Inc., where he helped guide the company’s transition from US$80 million market capitalisation (2008) to a multi-billion dollar group with the first approved drug for DMD now generating annual sales approaching US$400 million
Price’s initial focus will be on engaging with Key Opinion Leaders in the treatment of DMD, as well as DMD Patient Advocacy Groups to help increase the awareness of the company’s ATL1102 for DMD development program.
This will entail translating the features and benefits of the program to these audiences and to advocates internationally and in the capital markets.
Upon commencement of the company’s pivotal trial of ATL1102 in Europe, Price’s responsibilities will also include pharmacovigilance oversight, adverse event reporting and clinical safety monitoring.
Price has collegial support
Having worked with Price for more than eight years at Sarepta, William Goolsbee, non-executive director of Antisense Therapeutics said, “It is great news that Dr. Price has agreed to join our team.
‘’Accessing his deep understanding of DMD and his years of commercial development experience is a tremendous advance for our company.
‘’Gil's skill set, combining the views of a physician, the rigors of a clinical scientist, understanding of the Patient Advocacy community and experience in translating these things to the capital markets will be a huge assistance in bringing the ATL1102 to the US, the European Union and beyond.’’
Particularly with DMD, Antisense Therapeutics needs both scientific insight and a high sense of urgency to meet the unmet need of the boys and their families.
Price believes that Antisense has the opportunity to bring an unprecedented drug to treat DMD and possibly other diseases.
Importantly, the appointment further validates the company’s business prospects, and on this note managing Director Mark Diamond said, ‘’We see his appointment as a critical step for the company in broadening our international presence as we advance towards drug commercialisation.”
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.



